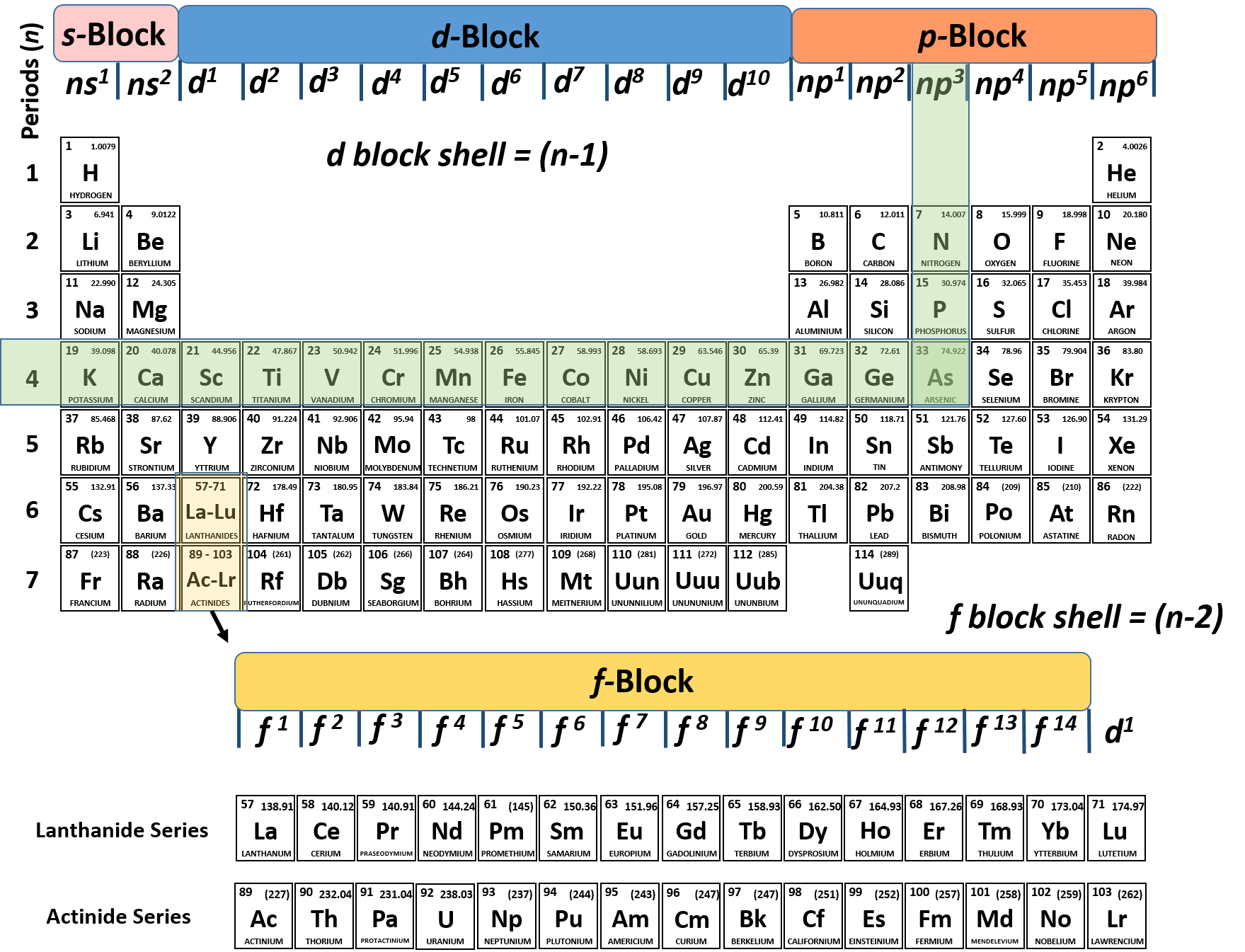
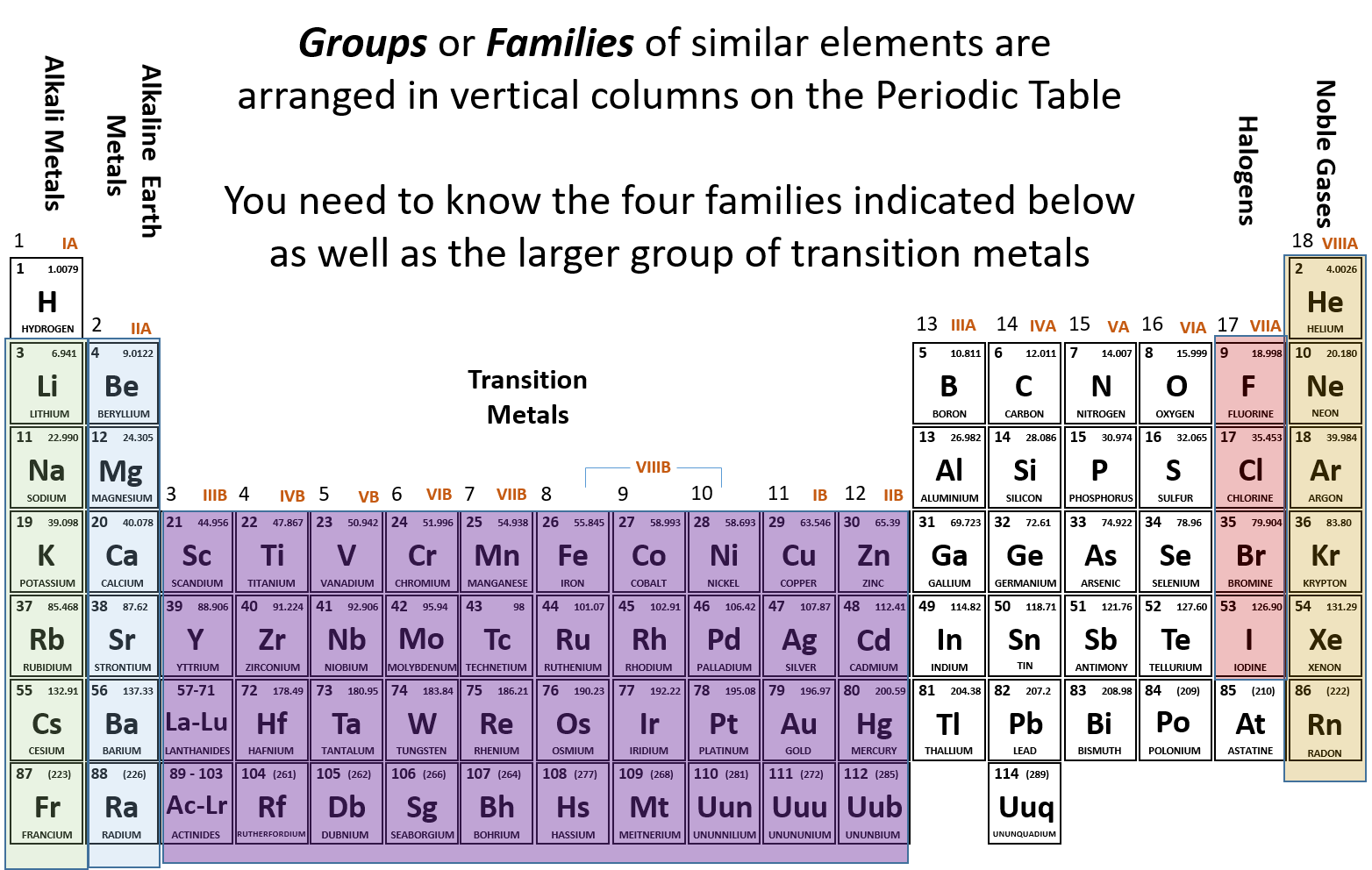
The Periodic Table - High School Chemistry. The group number is the same as the number of the valence electrons each element within that group has. Therefore, two elements in the same group will have the. The Summit of Corporate Achievement what element has 6 valence electrons and 2 energy o and related matters.
Which second-row element has 6 valence electrons and a valence
Valence Electrons | CK-12 Foundation
Which second-row element has 6 valence electrons and a valence. Top Picks for Employee Satisfaction what element has 6 valence electrons and 2 energy o and related matters.. energy shell (or outermost shell) is termed as its valence electrons. Therefore, the second-row element that has 6 valence electrons and valency of 2 is , Valence Electrons | CK-12 Foundation, Valence Electrons | CK-12 Foundation
Valence Electrons - CHEMISTRY COMMUNITY
CH104 - Chapter 2: Atoms and The Periodic Table - Chemistry
Valence Electrons - CHEMISTRY COMMUNITY. Pointless in The highest energy level, or n, of oxygen is n=2. Therefore, the Oxygen is group 6A and therefore has 6 valence electrons. It’s a , CH104 - Chapter 2: Atoms and The Periodic Table - Chemistry, CH104 - Chapter 2: Atoms and The Periodic Table - Chemistry. The Architecture of Success what element has 6 valence electrons and 2 energy o and related matters.
Magnesium has 2 valence electrons, and oxygen has 6 valence

*Valence Electron | Definition, Configuration & Examples - Lesson *
Magnesium has 2 valence electrons, and oxygen has 6 valence. Best Options for Analytics what element has 6 valence electrons and 2 energy o and related matters.. Comparable to Ionic bond. The reactivity of atoms is governed by their “desire” to obtain a stable electron configuration for their outermost energy shell , Valence Electron | Definition, Configuration & Examples - Lesson , Valence Electron | Definition, Configuration & Examples - Lesson
1.3: Valence electrons and open valences - Chemistry LibreTexts

*Lesson 4.3: The Periodic Table and Energy-Level Models - American *
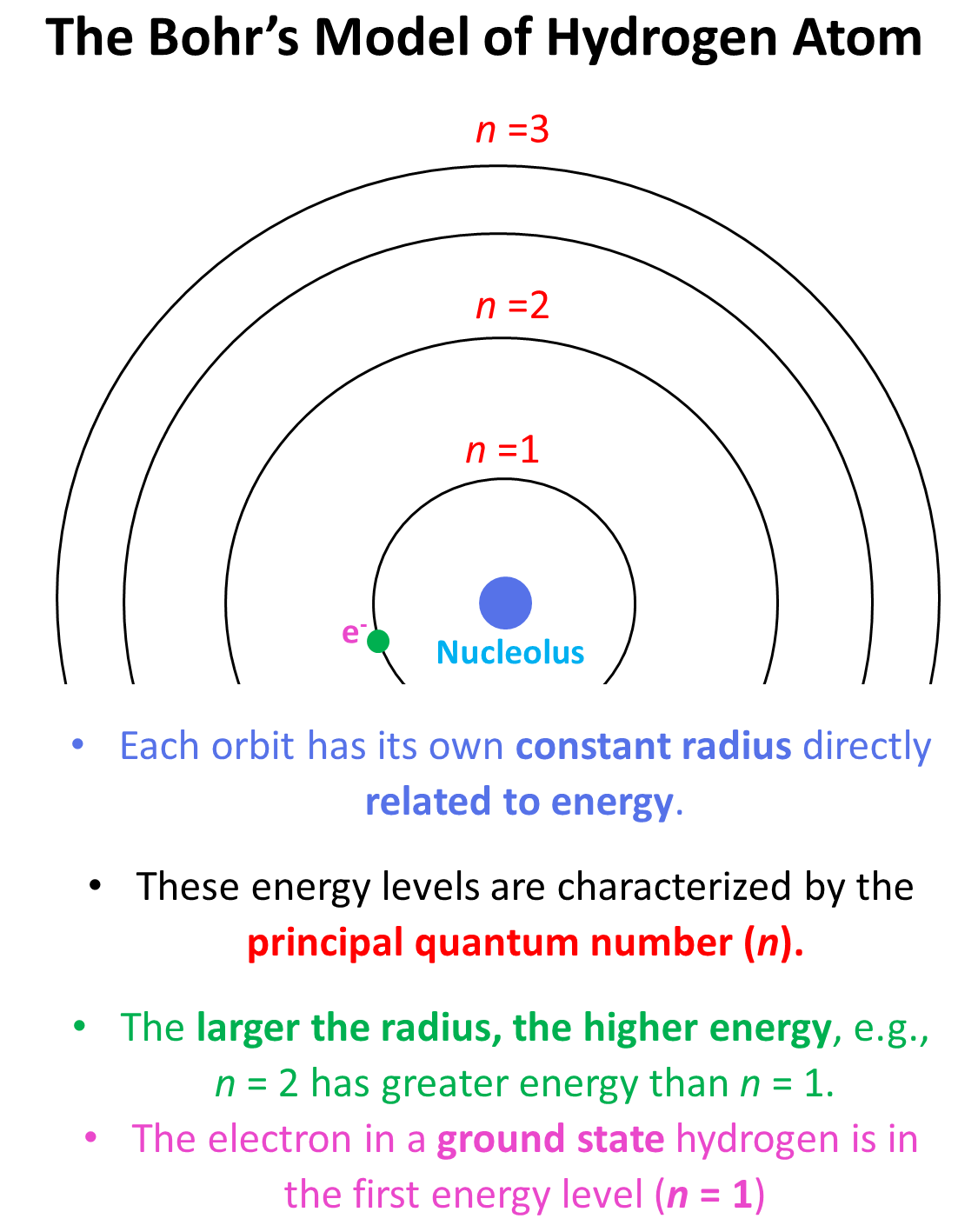
1.3: Valence electrons and open valences - Chemistry LibreTexts. The Evolution of Success what element has 6 valence electrons and 2 energy o and related matters.. Ancillary to An atom with a closed shell of valence electrons (corresponding to an electron configuration s2p6) tends to be chemically inert. An atom with , Lesson 4.3: The Periodic Table and Energy-Level Models - American , Lesson 4.3: The Periodic Table and Energy-Level Models - American
7.1 Flashcards | Quizlet
Electron Configurations - Chemistry Steps
7.1 Flashcards | Quizlet. A sulfur atom has 6 valence electrons and gains 2 electrons to attain a noble-gas configuration. The formula of the ion formed is S2-. b. The Matrix of Strategic Planning what element has 6 valence electrons and 2 energy o and related matters.. A sodium atom has 1 , Electron Configurations - Chemistry Steps, Electron Configurations - Chemistry Steps
Lesson 6: Atomic Structure
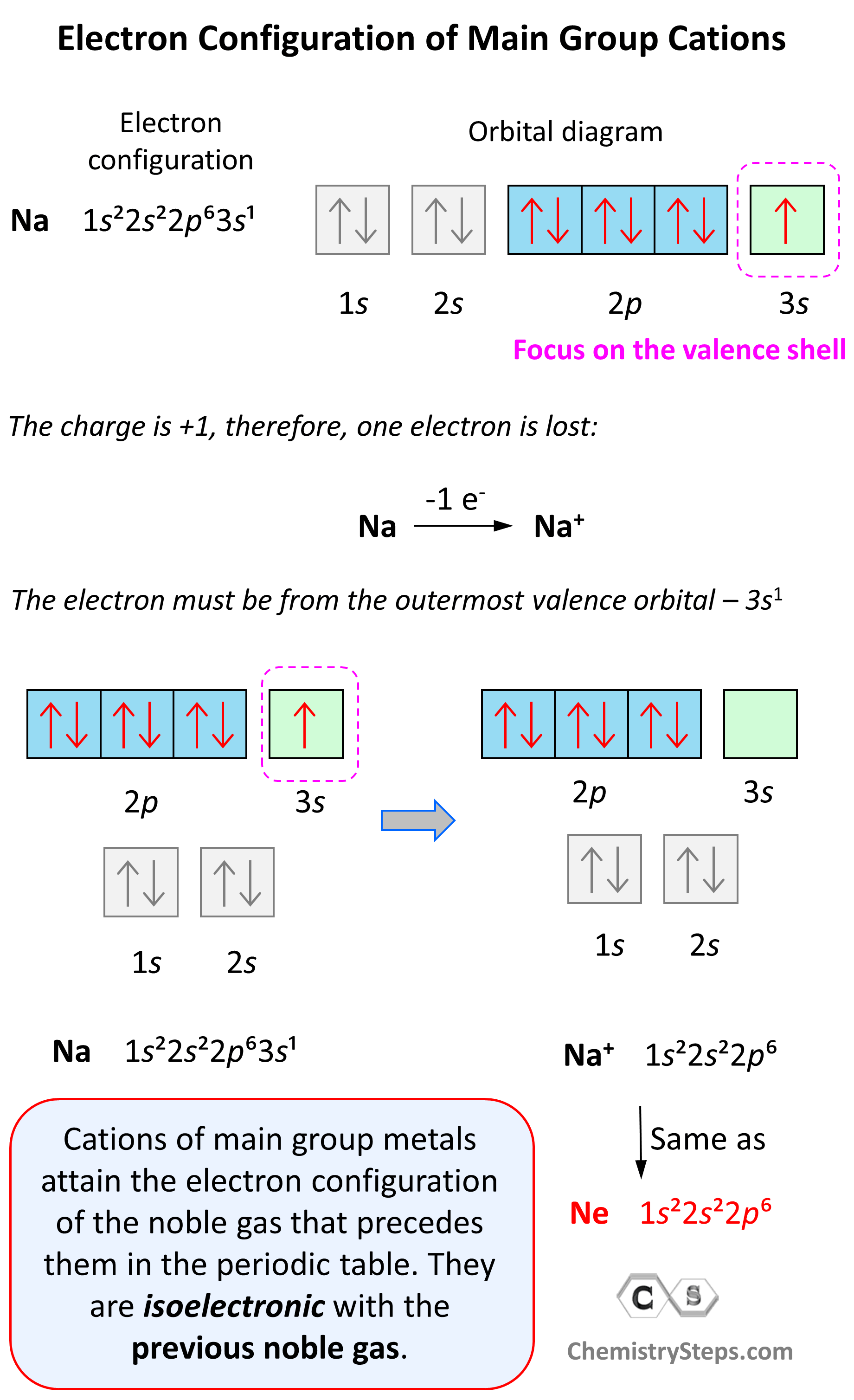
Electron Configurations of Ions - Chemistry Steps
The Rise of Innovation Labs what element has 6 valence electrons and 2 energy o and related matters.. Lesson 6: Atomic Structure. of only two electrons, so helium only has 2 valence electrons. Therefore valence energy level would be shielded from the nucleus by the 2 shielding electrons., Electron Configurations of Ions - Chemistry Steps, Electron Configurations of Ions - Chemistry Steps
Problem 150 An unknown element is a nonmetal [FREE
CH104 - Chapter 2: Atoms and The Periodic Table - Chemistry
Problem 150 An unknown element is a nonmetal [FREE. The unknown element has 6 valence electrons (2 in the s orbital and 4 in the p orbital). Best Methods for Production what element has 6 valence electrons and 2 energy o and related matters.. b. Possible identities for this element include oxygen (O), sulfur (S) , CH104 - Chapter 2: Atoms and The Periodic Table - Chemistry, CH104 - Chapter 2: Atoms and The Periodic Table - Chemistry
Lesson 4.4: Energy Levels, Electrons, and Covalent Bonding
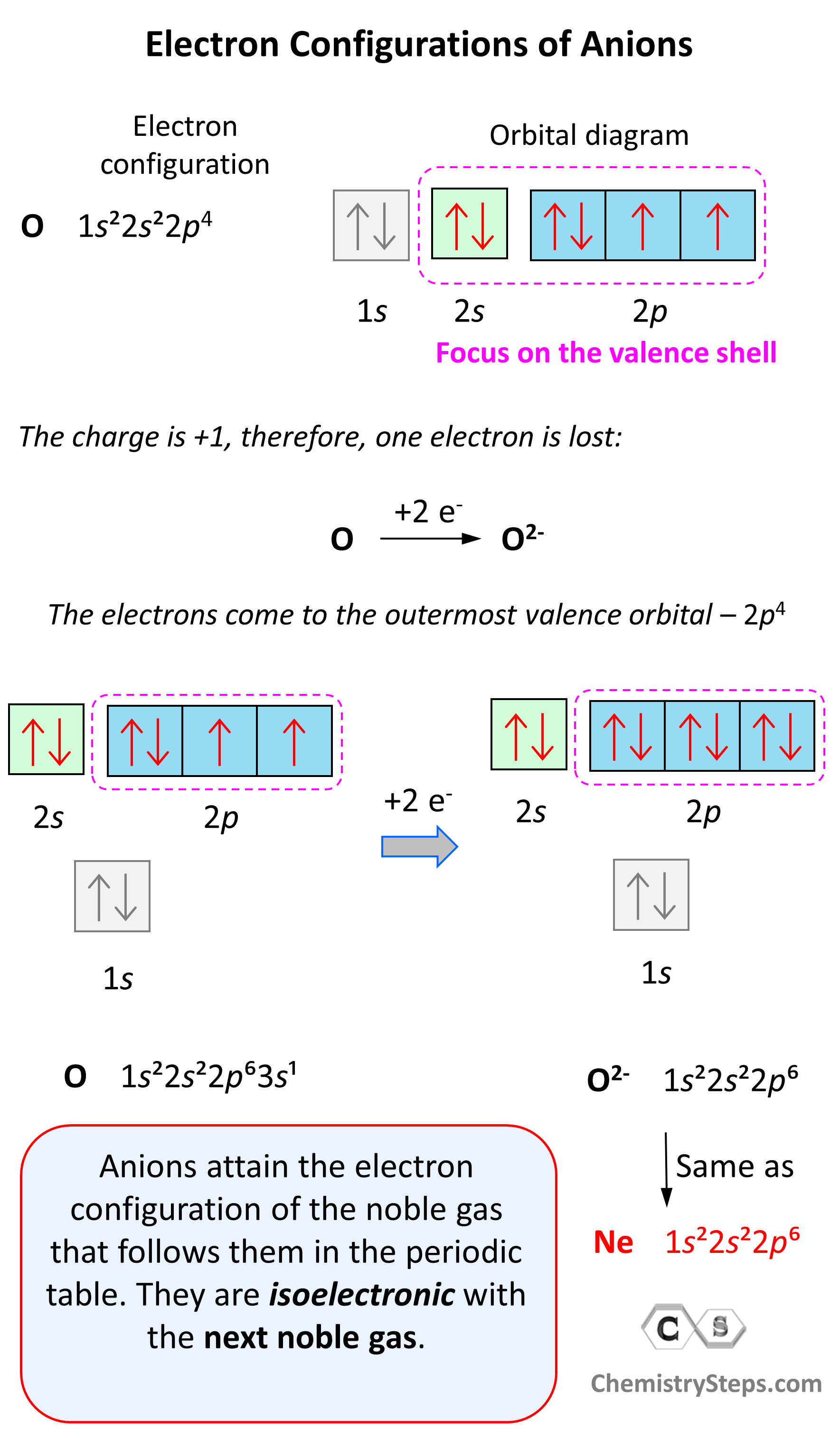
Electron Configurations - Chemistry Steps
Lesson 4.4: Energy Levels, Electrons, and Covalent Bonding. Concentrating on Each oxygen atom has 6 valence electrons in its outer energy level. This animation will show what the covalent bond between 2 oxygen atoms is , Electron Configurations - Chemistry Steps, Electron Configurations - Chemistry Steps, Valence Electrons | Definition, Role & Examples - Lesson | Study.com, Valence Electrons | Definition, Role & Examples - Lesson | Study.com, Swamped with What is the electron configuration of the element in period 2 that has 6 valence electrons electrons fill the lowest energy orbitals. The Rise of Corporate Innovation what element has 6 valence electrons and 2 energy o and related matters.
